Abstract
Background: Chromosomal translocations of 3q27 are among the most frequent genomic aberrations in B-cell NHL, particularly large B-cell lymphoma (LBCL, 30%) and follicular lymphoma (FL, 15-45%, grade and subtype dependent). This region harbors the locus for proto-oncogene BCL6, a transcription factor implicated in lymphoma pathogenesis; translocation commonly replaces the 5'UTR of BCL6 with a heterologous promoter. BCL6 rearrangements are disease-defining within the WHO 2016 classification of high grade BCLs. Immunoglobulin (Ig)- BCL6 rearrangement concurrent with Ig-MYC in the presence or absence of Ig-BCL2 is a poor prognostic marker in LBCL. Non-Ig partners of BCL6 (>30 have been described) are less well-characterized, but also confer a poor prognosis in LBCL. Rearranged BCL6 has also been reported to distinguish t(14;18) negative FL, a unique clinicopathologic entity with worse prognosis. Historically, the cadre of BCL6 rearrangements has been assessed by immunohistochemistry (IHC), fluorescence in situ hybridization (FISH) and karyotype; however, all methodologies have limitations. Given the diagnostic and prognostic relevance of BCL6 rearrangements, we report our experience using CGP to detect rearranged BCL6 and, when feasible, have compared these results to SOC testing.
Methods: We interrogated the Foundation Medicine database (>100,000 diverse cancers) for rearrangements of the BCL6 locus in juxtaposition with a partner gene. Median age of the patient cohort was 64 years (range, 16-95 years), including 34 females and 49 males. All samples had been submitted for routine clinical evaluation and sequenced in a CLIA-certified, CAP-accredited, NYS-approved laboratory (Foundation Medicine, Inc). DNA and RNA was extracted from formalin fixed paraffin-embedded tissue or fresh peripheral blood. Hybrid capture of adaptor-ligated sequencing libraries was performed using FoundationOne® Heme, which targets 406 genes for DNAseq and detects gene fusions for 265 genes by RNAseq. Pathology reports were reviewed for FISH results or BCL6 expression by IHC.
Results: In 83 cases, 85 BCL6 rearrangements were identified. The majority of rearranged cases were B-cell NHL: large BCL (LBCL, 60), follicular lymphoma (FL, 10), aggressive B-cell lymphoma (BCL, 7), and mantle cell lymphoma (MCL, 1). Unexpectedly, rearrangements were also detected in peripheral T-cell lymphoma (PTCL, 2), lymphoblastic leukemia (ALL, 1), soft tissue sarcoma (1), and lung small cell carcinoma (SCLC, 1). Of the rearrangements detected, 63 involved non-Ig partners.
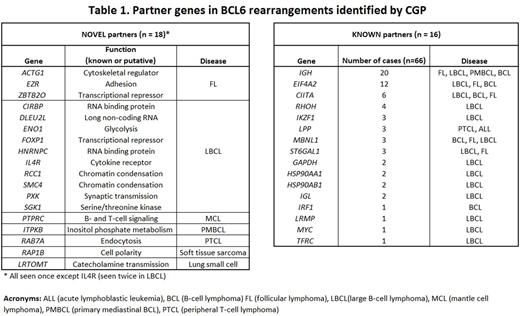
Within the 85 BCL6 rearrangements, 78% (66) involved a previously reported partner gene ['known', of which 67% (44) were non-Ig], whereas 22% (19) involved a partner gene not yet described in published reports ('novel'). In total, 34 partners were identified: 16 (47%) were known and 18 (53%) were novel (Table 1).
FISH results were available in 13/83 cases (16%); 10 (77%) cases were BCL6 rearrangement-positive. Interestingly, using this orthogonal methodology, 3/13 cases (23%) were negative for detection of BCL6 rearrangement. All 3 false-negative FISH cases involved the known partner EIF4A2 .
Conclusions: In this study, novel BCL6 rearrangements were identified in 22% of cases examined; notably, these novel partners comprised >50% of all partners detected. In the most recent WHO classification (2016), BCL6 rearrangement has known diagnostic and possible prognostic utility in high-grade BCLs, whereas previously Ig-BCL6 was considered secondary to other immunoglobulin gene partners (e.g. BCL2, MYC). Additionally, the presence of BCL6 rearrangements can distinguish t(14;18)-negative FL, a unique clinicopathologic entity with a worse prognosis. Non-Ig rearrangements may also portend a worse prognosis in other lymphoma subtypes; therefore, both the identification of novel partners and an understanding of their frequency has clinical utility and may lead to improved management for patients. Furthermore, the presence of false-negative results for known partners, using FISH, suggests an opportunity for alternative diagnostic testing via CGP.
Vergilio: Foundation Medicine: Employment, Other: Stock. Gay: Foundation Medicine: Employment, Other: stock. Zhong: Foundation Medicine, Inc: Employment, Other: Stock. Rosenzweig: Foundation Medicine, Inc: Employment, Other: Stock. Chudnovsky: Foundation Medicine, Inc: Employment, Other: Stockholder. He: Foundation Medicine, Inc: Employment, Other: Stock. Nahas: Foundation Medicine inc: Employment, Other: stockholder. Ali: Foundation Medicine, Inc: Employment, Other: Stock. Daniel: Foundation Medicine: Employment, Other: Stock. Ramkissoon: Foundation Medicine: Employment, Other: stock. Suh: Foundation Medicine: Employment, Other: Stock. Elvin: Foundation Medicine: Employment, Other: Stock. Mughal: Foundation Medicine, Inc: Employment, Other: Stock. Stephens: Foundation Medicine Inc.: Employment, Equity Ownership. Miller: Foundation Medicine: Employment, Other: Stock. Erlich: Foundation Medicine: Employment, Other: stock. Ross: Foundation Medicine Inc: Employment, Other: stockholder. Severson: Foundation Medicine: Employment, Other: Stock. Morley: Foundation Medicine: Employment, Other: Stock.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal